Ever wondered how a person with zero chemistry knowledge can influence public health? Enter Vani Hari, aka The Food Babe.

Sage advice from Vani Hari, aka The Food Babe.
This is quite a theory, enough so to examine what she means, whether there is anything to it, and how this may pertain to allergic reactions to Vitamin B12. Hang in there. This sounds like Crazyville, but it will make sense.
Hari's knowledge of chemistry exists only in her mind. The "pronunciation test" is patently ridiculous. In Hari World, Vitamin B12 is either safe or toxic—depending entirely on which of its names you choose.
And just to keep things relatively sane, a little science. I'll show you (below) why 1% of you have B12 allergies.
The silliness of the pronunciation test.
I've written many times that the nomenclature of chemistry is a verified madhouse. Want proof? This should do. The following are all legitimate names for aspirin.

Different names for aspirin. Madness. Source: ChemSpider
Some (but not all) names for vitamin B12
To be fair to Hari, I do not know one organic chemist who is even remotely capable of coming up with a chemical structure based on this name and many would have trouble pronouncing it. The following monstrosity is the IUPAC name (1) for vitamin B12.
Cyano-[(1R,2S,3S,4Z,7S,8S,9Z,13S,14Z,17R,18R,19R)-2,7,18-tris(2-amino-2-oxo-ethyl)-3,8,13-tris(3-amino-3-oxo-propyl)-17-[3-[[(2R)-2-[[(2R,3S,4R,5S)-5-(5,6-dimethylbenzimidazol-1-yl)-4-hydroxy-2-(hydroxymethyl)tetrahydrofuran-3-yl]oxy-oxido-phosphoryl]oxypropyl]amino]-3-oxo-propyl]-1,2,5,7,12,12,15,17-octamethyl-8,13,18,19-tetrahydro-3H-corrin-21-yl]cobalt(1+)
The IUPAC name for vitamin B12. In some language or other.

Let's try a different name that Hari may or may not be able to pronounce: Cyanocobalamin. Regardless of its pronounceability, it sounds pretty scary, right?
No. It's not. But here's why it may seem so.
First, CYANOcobalamin, pronunciation notwithstanding, sure sounds a lot like CYANIDE, the go-to poison in many murder mysteries. And cyanoCOBALamine (COBAL being short for the metal cobalt) doesn't sound so great either. Cobalt has similar properties to heavy metals (2).
Finally, let's try this one: Vitamin B12. Quite pronounceable and safe, leading to the inescapable conclusion that it either should or should not be avoided, depending on which name one chooses to identify it.
Vitamin B12
Quite pronounceable and safe, leading to the inescapable conclusion that it either should or should not be avoided, depending on which name one chooses to identify it. Make sense? Didn't think so. Let's move on to something a bit more useful.
A brief, possibly even useful, lesson on B12
Although cobalt-containing biomolecules are rare in nature, cyanocobalamin is not; it is found in all living creatures. It is also structurally very similar to two other common organometallic (2) molecules, heme, and chlorophyll (Figure 1), all of which are critical biomolecules for animal and plant life.
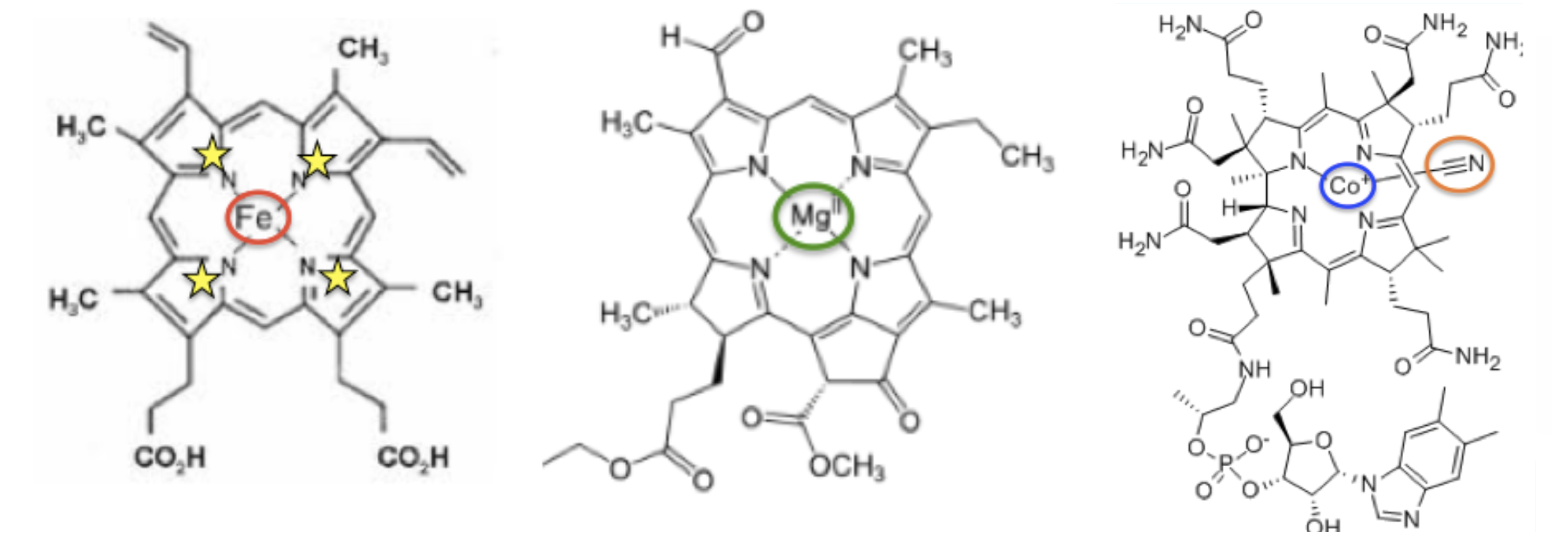
Figure 1. Biologically important metal coordination complexes: (Left) Heme, the "business end" of hemoglobin. Iron (red circle) carries oxygen throughout the body. (Center) The central structure of chlorophyll. Magnesium (green circle) enhances light absorption. (Right) Cyanocobalamin, one of the forms of Vitamin B12 .Cobalt (blue circle) is required for DNA synthesis and fatty acid metabolism.
Note that in all three molecules metal ions are coordinated - held in the middle - of a structure that contains four nitrogen atoms (yellow stars) which are part of five-membered rings that are geometrically perfected to accommodate metals in the middle. . This motif is nature's way of binding organic metals to metals.
Why the allergies?
Since vitamin B12 is essential for every cell in the human body (animals too), it may seem strange, that some people are allergic to it and they can suffer either an acute or chronic itchy rash that can have small blisters containing a clear fluid, skin that is red, swollen, oozing, or peeling. A cobalt allergy can be confirmed by a skin patch test (Figure 2).
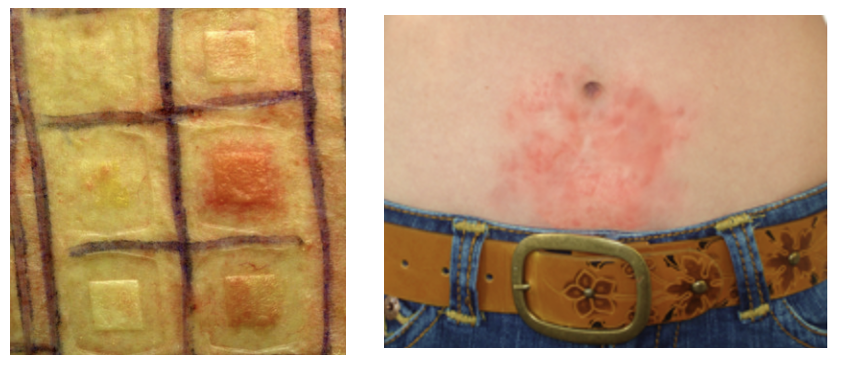
Figure 2. (Left) A typical skin patch test characteristic of a nickel allergy. Source: Wikimedia Commons. (Right) Nickle/cobalt allergic dermatitis. Source: Allergy Best Buys
Why would anyone be allergic to cobalt? Chemistry gives us an answer. Figure 3 shows that nickel – a well-known allergin – resides right next to cobalt on the periodic table. The two metals have similar properties, so a person who is allergic to nickel may also be allergic to cobalt.
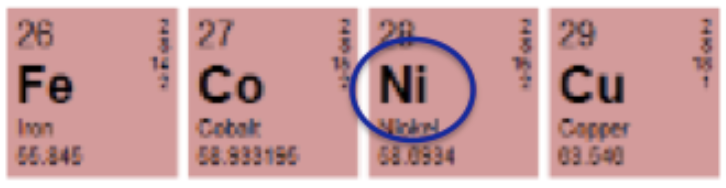
Figure 3. Cobalt has an allergic neighbor
"Nickel allergy is a common cause of allergic contact dermatitis — an itchy rash that appears where your skin touches a usually harmless substance... It may take repeated or prolonged exposure to items containing nickel to develop a nickel allergy. Treatments can reduce the symptoms of nickel allergy. Once you develop a nickel allergy, however, you'll always be sensitive to the metal and need to avoid contact."
Mayo Clinic
Bottom line
People who are allergic to B12 are reacting to the presence of cobalt – a metal similar to nickel nickel, which is a known allergen. What do people who are B12 deficient but also allergic to cobalt do? They cannot avoid the cobalt since it is part of the vitamin, which they cannot do without it. The general advice seems to be "take just enough but not too much."
This advice isn't too different from dosing advice for all vitamins and drugs. Whether this holds true for coffee enemas, celery juice cleanses, or Dr. Oz's magic fat burners remains to be seen. We live in interesting times.
NOTES:
(1) Organometallic chemicals contain at least one covalent bond between a carbon atom and a metal. Some are stable and benign, some ignite when exposed to air, and one of them is so dangerous that it killed a chemist who spilled a tiny amount on the back of her glove. (See Two Drops Of Death: Dimethylmercury)
(2) Heavy metals are dense metallic elements like lead, mercury, and arsenic that can be toxic to humans and the environment even at low concentrations. Some, like iron and zinc, are essential for life.



