For nearly 80 years, fluoride has quietly flowed through our water systems, credited as one of the 20th century’s public health victories. RFK, Jr. promises to challenge water districts on fluoridation, turning that legacy on its head. The debates swirl around health, ethics, and legal liabilities, and we owe ourselves understanding both viewpoints based on more than a soundbite or a headline.
“I am going to advise the water districts about their legal liability, their legal obligations, their service to their constituents, and I’m going to give them good information on the science and fluoride will disappear.”
- RFK, Jr.
With RFK, Jr. possibly ascending to be the head of Health and Human Services, it is entirely possible that fluoridation as we know it may disappear. Fluoridation has been with us for about 80 years; enough time has passed that few have a living memory of the time before community water fluoridation. The federal government is not in charge of our water supply; that is an issue of local governance. This is why ACSH is running an episodic series on fluoridation: to give you the history, science, and legal wrangling so you can be a better-informed citizen.
Mechanisms of Action of Fluoride for Caries Control
Dental caries, cavities, are a result of our oral environment – which includes its bacterial biome, dental biofilms, saliva, the food we eat, and genetics to some greater or lesser degree. Fluoride has been a pivotal adjunct in the war against cavities and is widely recognized as the main factor responsible for the global decline in the prevalence of caries. The underlying mechanism of fluoride’s impact involves both systemic and topical effects. Which of the two is believed to be the prime mover in preventing decay has gone back and forth over the years.
Caries
Enamel is a tissue without cells (acellular) composed primarily of calcium-deficient
carbonated hydroxyapatite forming its structural matrix. Dentin, which lies further down in the gum line, contains cells and lesser amounts of hydroxyapatite, and its structure is a combination of the matrix formed by the mineral and collagen produced by the cells. Caries are the net result of cycles of de- and remineralization of these dental tissues.
Caries in the enamel result solely from a chemical reaction; dentin’s response is a biochemical process – involving both that chemical reaction and enzymatic breakdown by oral bacteria. While neither enamel nor dentin is fully resistant to caries, enamel, affected solely by chemistry, is more resistant than dentin.
Our saliva contains all the chemical elements necessary for the formation and breakdown of hydroxyapatite, the scaffolding of our teeth. The combination of the acidity within the mouth, measured as pH, and a variety of enzymes controls the breakdown of hydroxyapatite (demineralization) or its formation (remineralization). The bacteria within our mouths, our oral microbiome, especially when fed sugar, generate lactic acid, lowering the pH and enhancing demineralization. The critical value for hydroxyapatite is a pH of 5.5 – below that hydroxyapatite breaks down.
Fluoride
Lowering the mouth’s pH to 5.5 or less in the presence of fluoride favors the chemical conversion of hydroxyapatite to fluorapatite. 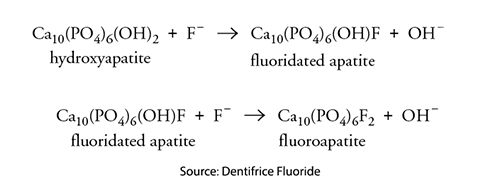 However, the critical pH for fluorapatite demineralization is 4.5. – a level of acidity that is less commonly reached within our mouth. As a result, enamel and dentin, with more fluorapatite, are more resistant to demineralization and the formation of cavities. An unfortunate side effect of this substitution is that fluorapatite crystals also impede remineralization. Over time, the increasing presence of fluorapatite becomes visible as discolorations of the enamel, dental fluorosis.
However, the critical pH for fluorapatite demineralization is 4.5. – a level of acidity that is less commonly reached within our mouth. As a result, enamel and dentin, with more fluorapatite, are more resistant to demineralization and the formation of cavities. An unfortunate side effect of this substitution is that fluorapatite crystals also impede remineralization. Over time, the increasing presence of fluorapatite becomes visible as discolorations of the enamel, dental fluorosis.
Systemic fluorides are those we ingest and deposit throughout enamel and dentin, providing long-lasting protection. They also offer topical protection because they are present in our saliva, creating a reservoir of fluoride. Systemic fluorides come from our community water supply, dietary supplements and are present in our food and beverages, including bottled water.
Topical fluorides are transient and are found in products we associate with oral hygiene, e.g., toothpaste and mouthwash. A small part of topical fluorides may be swallowed, adding to our systemic supply.
 The earliest fluoride studies were prompted by the fluorosis in children’s teeth and fluorine’s preventative impact on caries was a serendipitous finding. Those studies were all based on fluoride levels in the water. There were no other sources of fluoride, so the intuition that fluoride’s effect was primarily systemic made sense. Ingestion of fluoride resulted in tissues involved with tooth formation, as well as saliva, being enriched in fluoride.
The earliest fluoride studies were prompted by the fluorosis in children’s teeth and fluorine’s preventative impact on caries was a serendipitous finding. Those studies were all based on fluoride levels in the water. There were no other sources of fluoride, so the intuition that fluoride’s effect was primarily systemic made sense. Ingestion of fluoride resulted in tissues involved with tooth formation, as well as saliva, being enriched in fluoride.
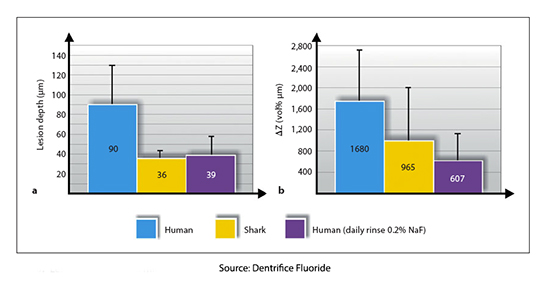
It was only in the 1980s that an experiment demonstrated that fluoride in saliva was the primary driver, more than tooth progenitors. Researchers took “slabs” of human and shark tooth enamel, which has 30,000 times more fluorapatite than humans, and bathed them in an in vitro environment. Shark teeth were far more resistant to caries seen on micrographs than humans until the human enamel was bathed in a solution of fluoride, at which point human and shark resistance were equal.
The argument could then be made that topical fluoridation, from drinking fluoridated water or brushing our teeth with fluoride-containing toothpaste, is sufficient to prevent caries.
There is one fly in the toothpaste, as it were. There remains good evidence that fluoride has a beneficial effect on the development of caries in teeth yet to have “erupted” and that have not been exposed to topical fluoride. That suggests that systemic fluoride incorporated into these teeth as they are formed is protective. This belief is further bolstered by fluoride’s protection of dentin, which is more susceptible to acid attack than enamel, demineralizing more quickly and deeply and remineralizing more slowly. It requires a higher amount of fluoride to gain the same protection. Because dentin is further down in the gum line, it is less exposed to topical fluorides, an argument for adults using fluoride in drinking water.
As the author of Dentifrice Fluoride ends,
“Knowledge of the mechanisms by which fluoride promotes caries control is essential for the achievement of the maximum benefits of this element with minimum risk of side effects.”
Whether systemic and topical fluorides are both necessary to most effectively prevent tooth decay is unknown, but that is the question that should drive the debate around the continued fluoridation of water. Fluoride’s caries-fighting power is undeniable. RFK, Jr.’s vision to dismantle fluoridation, however intentioned, threatens, without conclusive evidence, the public health gains it has secured.
Sources: Mechanisms of Action of Fluoride for Caries Control
Dentifrice Fluoride Chemistry for Everyone Journal of Chemical Education DOI: 10.1021/ed081p677
Systemic versus Topical Fluoride Caries Research DOI: 10.1159/000077764



